
Regulatory Compliance
Let's break down the six most important regulations and frameworks for healthcare cybersecurity. 1. HIPAA Security Rule. You already know about HIPAA, but its Security Rule is arguably the most important regulation for healthcare cybersecurity. If you're a covered entity or a business associate of a covered entity, you have to follow the.
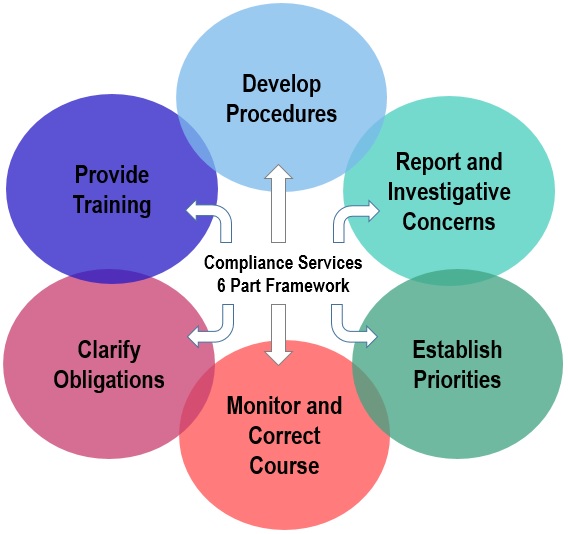
Compliance Services Office Program Framework » Compliance Services Boston University
A Compliance Framework for the U.S. Healthcare Industry. If you operate outside the U.S. and handle patient and other sensitive data, the legal obligation to comply with U.S. law extends to you. Specifically, the U.S. healthcare compliance framework serves these purposes:

The International Health Regulations 10 years on the governing framework for global health
Healthcare Compliance Requirements. That healthcare compliance is a tricky task would be a tremendous understatement. As mentioned earlier, simply understanding which regulations an organization is subject to and must comply with can be arduous, as multiple federal regulations overlap in terms of healthcare requirements.

Importance of Regulatory Compliance & Risk Management AK Enterprizes (The Learning Lab)
Regulatory compliance laws for healthcare organizations and professionals are developed to protect the private information of patients when it comes to personal, medical history, and payment information. It also outlines the requirements to ensure quality patient care and to combat fraud within healthcare organizations.

Standards Vernance
On Monday November 6, 2023, the US Department of Health and Human Services, Office of Inspector General (OIG) released its new General Compliance Program Guidance (GCPG). The GCPG provides voluntary compliance guidelines and identifies risk areas that OIG believes healthcare industry participants should consider when developing and implementing a new compliance program or evaluating and.
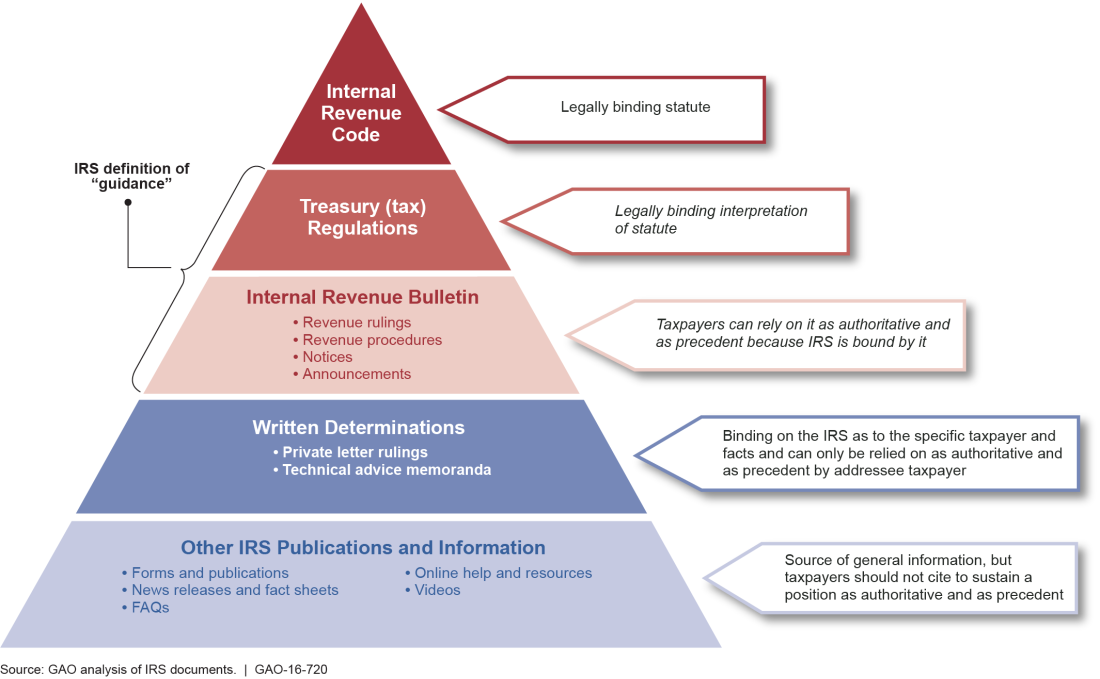
Regulatory Guidance Processes Treasury and OMB Need to Reevaluate Longstanding Exemptions of
Introduction. The delivery of high quality care is a fundamental goal for health systems worldwide. Quality is variable, due to structural issues such as insufficient staffing levels [], or process issues like poor cleaning practices [], and can cause differences in outcome across health and social care providers such as high complication rates and poor patient experience [].

Creating A Healthcare Regulatory Compliance Plan
Healthcare is one of the most regulated industries in the United States, making healthcare compliance a crucial and growing field within the industry. The Bureau of Labor and Statistics projects the overall need for compliance officers to grow by over 8% from 2016 through 2026. Healthcare compliance professionals are needed to help clinical.

Government Rules And Regulations
With this in mind, here are some of the most important regulatory standards and compliance frameworks you need to be aware of. 1. Sarbanes-Oxley Act (SOX) The Sarbanes-Oxley Act (SOX) is one of the main regulations in the United States for those working with financial details in public firms. Passed in 2002 in the wake of scandals such as Enron.
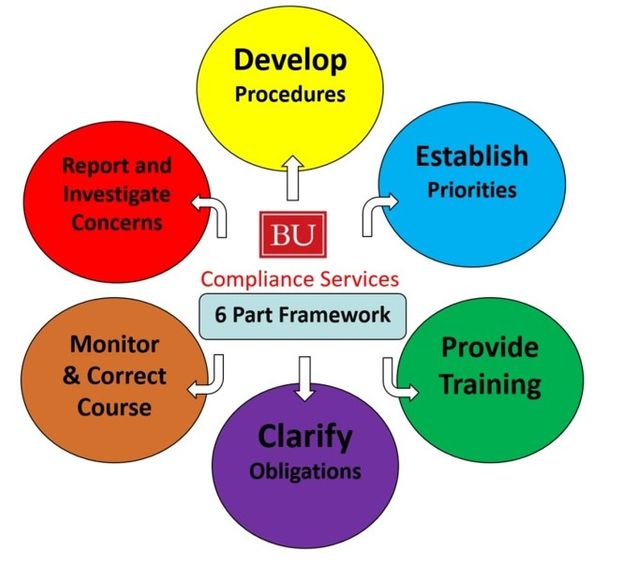
Compliance Framework » Compliance Services Boston University
The Health Insurance Portability and Accountability Act, or HIPAA, is one of the best known regulatory compliance frameworks among consumers in the United States. Introduced in 1996, it sets various standards and requirements regarding health data, among other things. HIPAA is relatively high-level and was introduced at a time when technology.

compliance_pic The Graduate Medical Education Compliance Project (GMECP)
Which compliance framework governs requirements for the U.S. healthcare industry? 1.FedRAMP, 2.GDPR, 3.PCI-DSS, 4.HIPAA
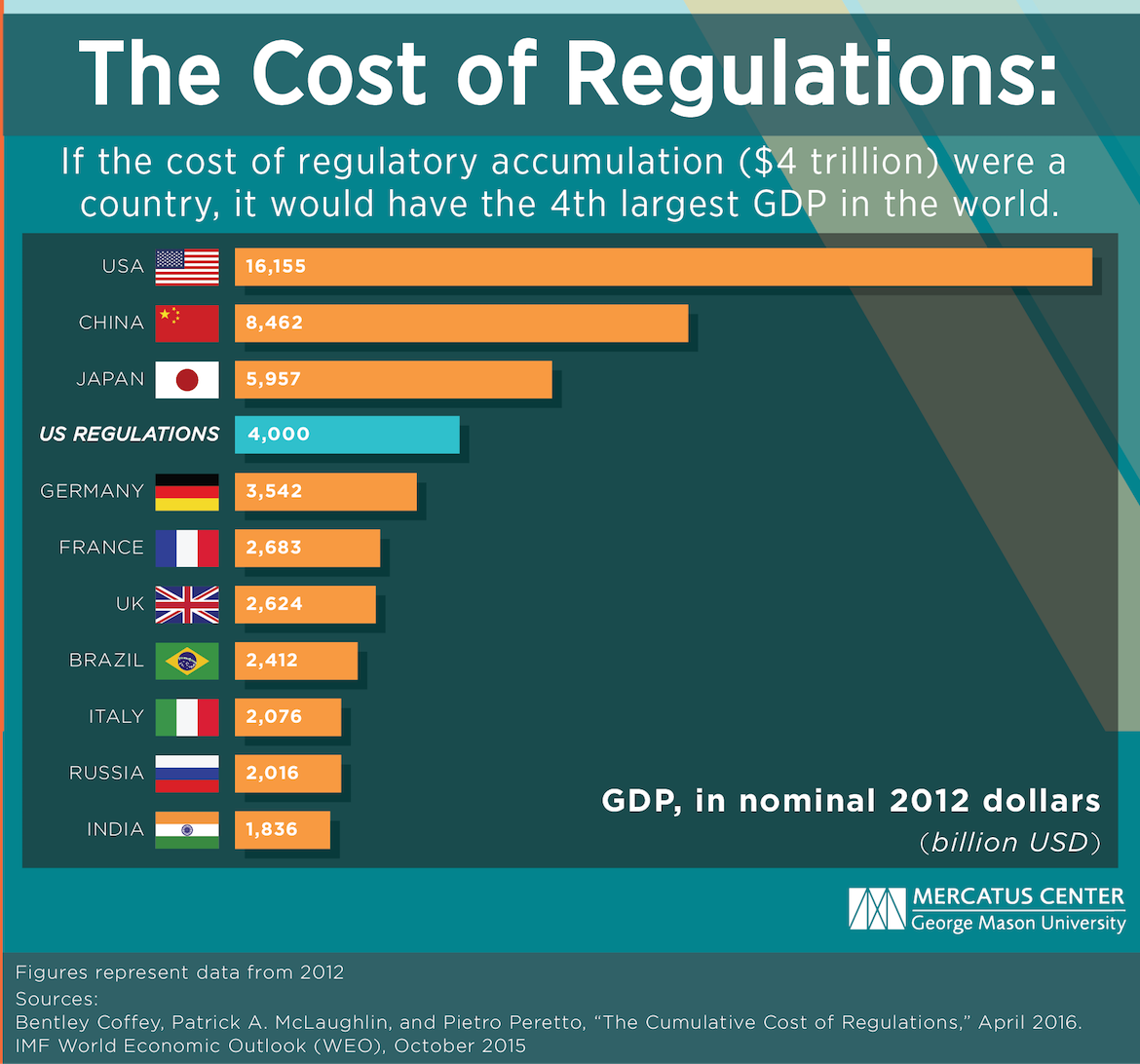
The Regulatory Burden in the U.S. is a Whopping 4 Trillion
There are various compliance frameworks that organizations can use depending on their industry, size, and specific compliance requirements. Some of the commonly used frameworks include: ISO 27001: This framework outlines the requirements for information security management systems. Businesses and organizations of all sizes and types can benefit.
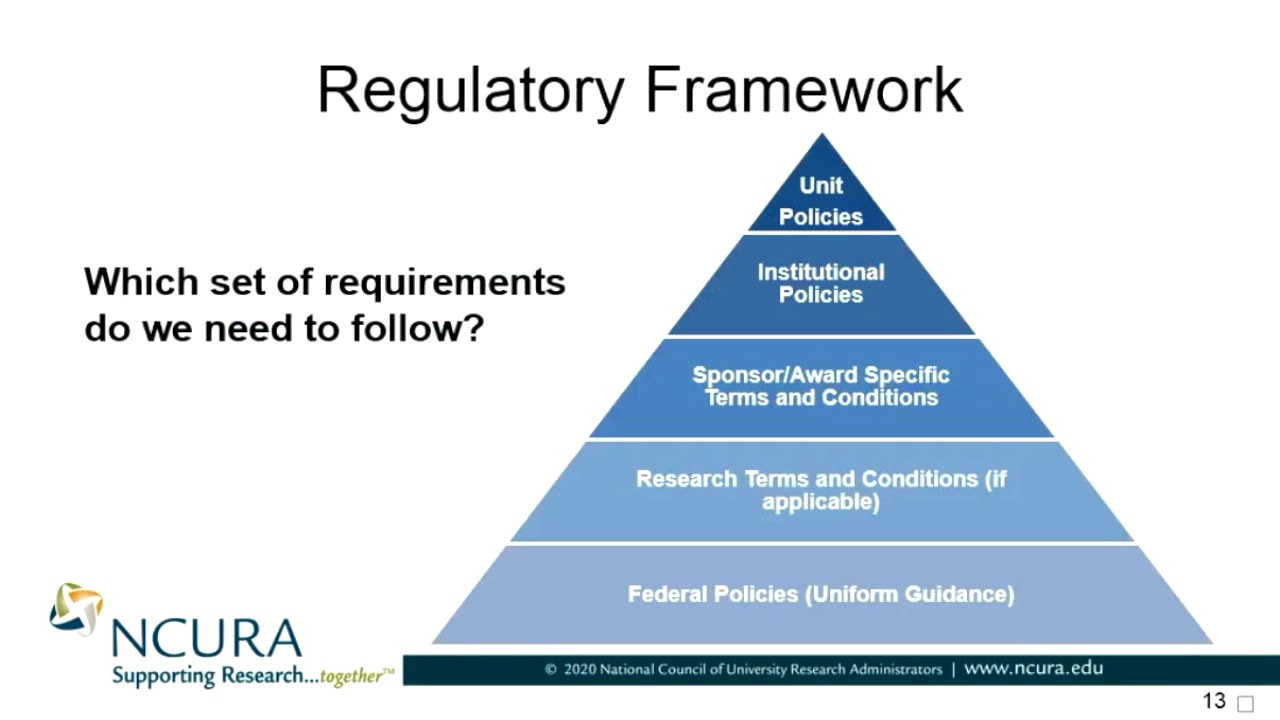
Regulatory Framework Which Set of Requirements Do We Need to Follow? YouTube
OIG's compliance documents include special fraud alerts, advisory bulletins, podcasts, videos, brochures, and papers providing guidance on compliance with Federal health care program standards. OIG also issues advisory opinions, which cover the application of the Federal anti-kickback statute and OIG's other fraud and abuse authorities to the.
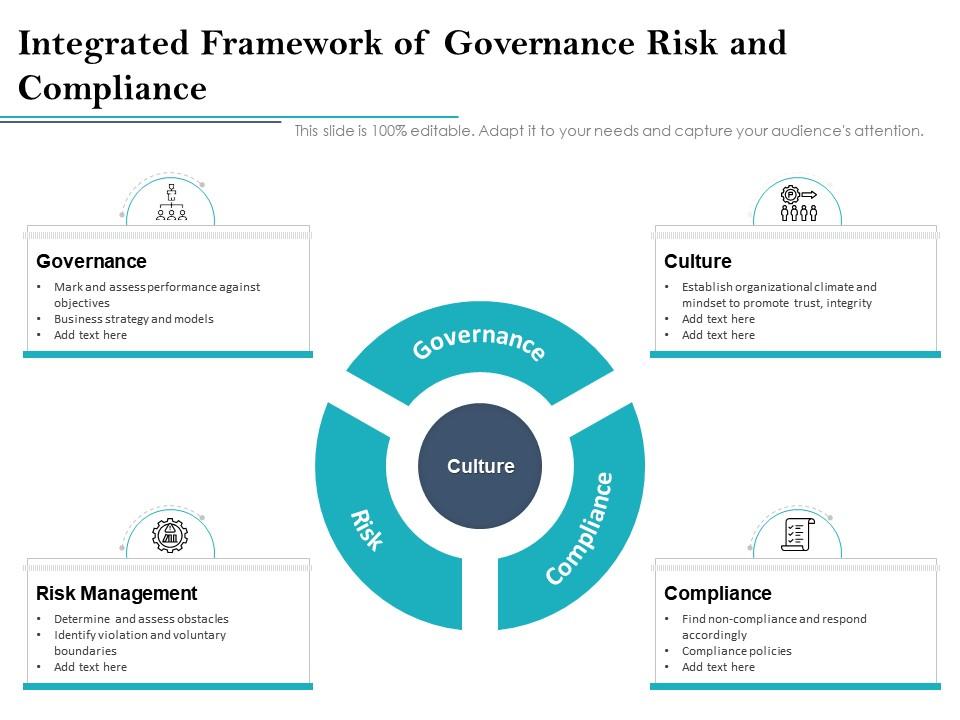
Integrated Framework Of Governance Risk And Compliance Presentation Graphics Presentation
Overview. The US healthcare industry is rigorously monitored against stringent regulatory norms. The industry needs to comply with a variety of standards including the Health Insurance Portability and Accountability Act (HIPAA), Centers for Medicare and Medicaid Services (CMS), American Society for Testing and Materials (ASTM), International Conference on Harmonization (ICH), International.

6 Government Regulations Business Owners Really Should Know
Further, healthcare professionals are governed by state professional licensure and related ethical requirements, the specifics of which may vary from state-to-state, and in addition the American Medical Association ("AMA") Code of Medical Ethics provides guidance regarding industry-provided gifts and subsidies for physicians. Further.
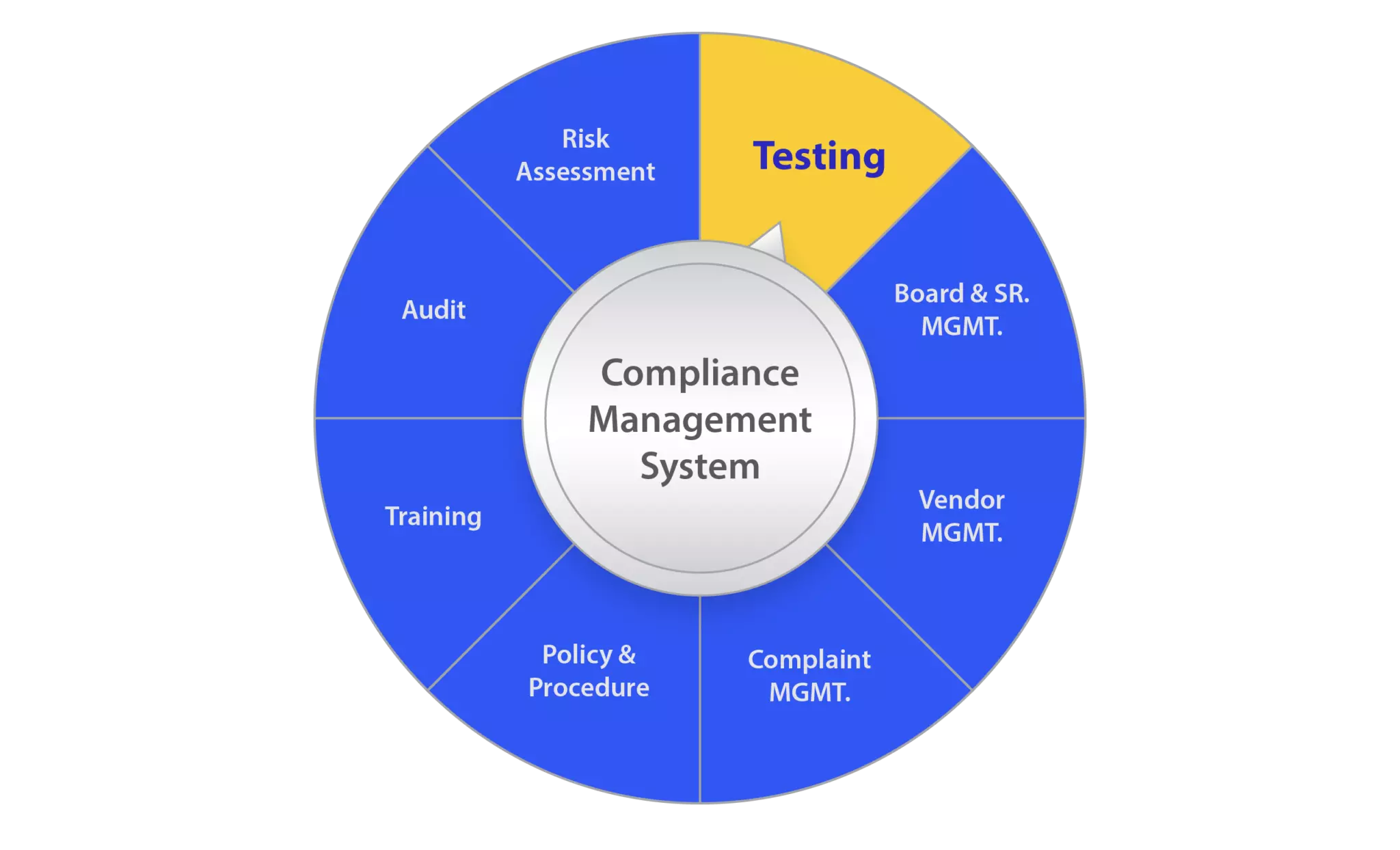
Regulatory Compliance Consulting NMS
Healthcare compliance frameworks are similar. There are a group of governing bodies (classes) that regulate healthcare compliance (the degree), and within each body are a set of requirements (projects) you need to complete. A compliance program, or framework (syllabus) is what you need to follow in order to achieve regulatory compliance for.

The Importance of Healthcare Compliance Programs ERD
PHI is defined in 45 CFR § 164.501. Covered Entity as defined in 45 CFR 160.103 is required to take necessary steps to ensure compliance with these HIPAA required ("R") clauses and addressable ("A") clauses. Covered Entities are mandated to comply with HIPAA required requirements but do not provide any specific framework.
- Botas De Montaña Quechua
- Best Parts Of Camino De Santiago
- 100 Peliculas Mas Taquilleras De La Historia
- Almacenamiento Lleno Hotmail Que Hacer
- Toyota Aygo 800cc Price In Pakistan
- Rugby Union Teams In England
- La Casita Del Pan Granada
- Ejercicio De Traducción Cerezo Rosa Ramon Solsona Llibreta De Vacances
- Vestidos De Fiesta Largos De Terciopelo
- Melia Jardines Del Teide Habitaciones
